Barrick Lab :: Research
Listen to an introduction to our research: Studying Evolution and Engineering Bee Guts (EBRC in Translation Podcast)

See also: Previous Research Projects
Evolving and Engineering Insect Symbionts
Many insects have more consequential associations with bacteria than we have with the human microbiome. We create genetic tools to study these bacterial symbionts and engineer their interactions with insects. We also use experimental evolution to examine how new symbionts arise and how interactions within a microbiome and between microbes and their hosts can change. These projects are highly collaborative and have also involved researchers from the Moran Lab, the Ellington Lab, the Davies Lab, and other research groups.
![]() Current Funding: ARO MURI, NSF EDGE, ERDC Past Funding: DARPA BRICS, DARPA Insect Allies
Current Funding: ARO MURI, NSF EDGE, ERDC Past Funding: DARPA BRICS, DARPA Insect Allies
![]() Overview Publication
Overview Publication
- Elston et al. (2022) Trends in Microbiology PMID: 34103228
Honey Bees: Functional Genomics and Protecting Pollinator Health
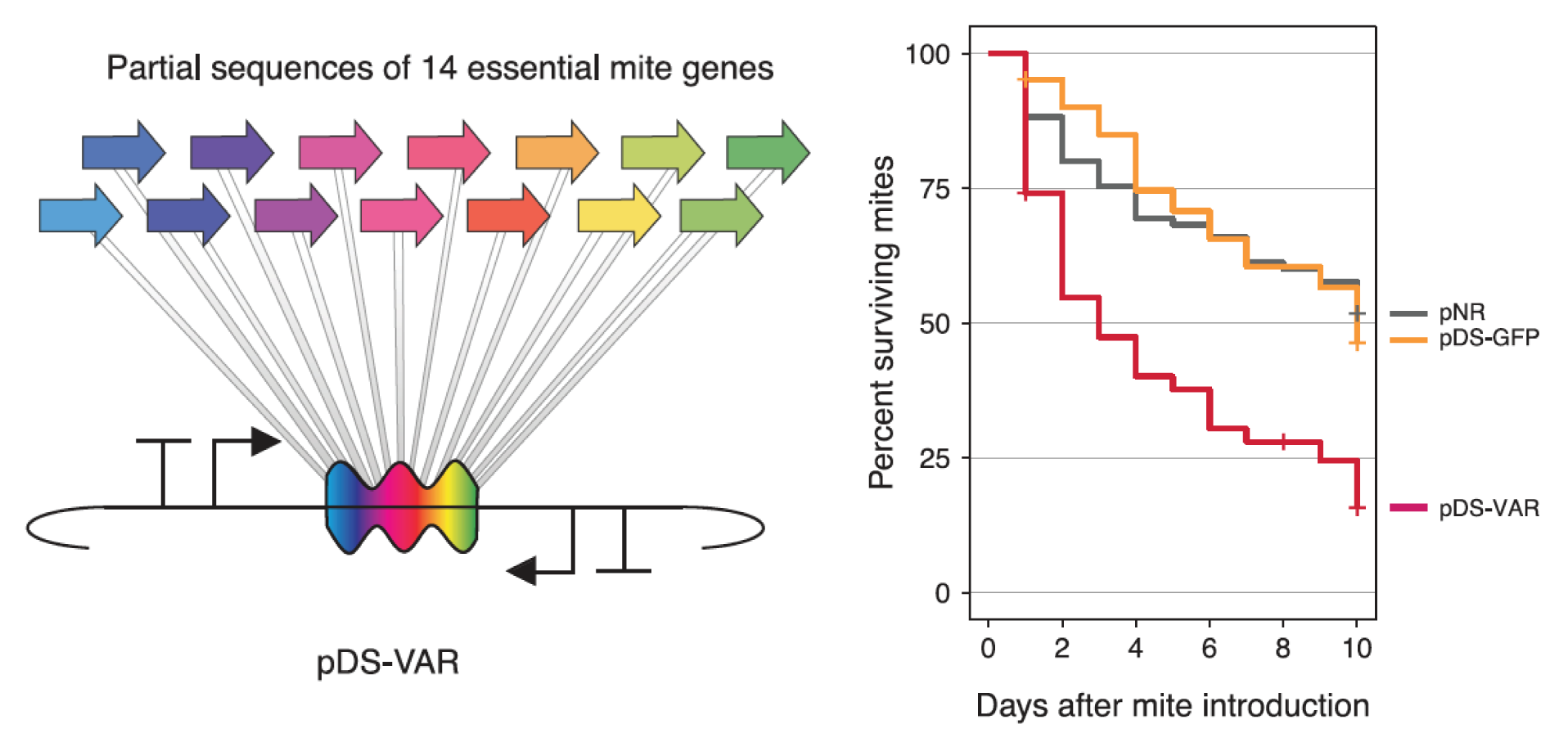
Engineered symbionts kill honey bee parasites
![]() Current Researchers: Zuberi Ashraf, Kadena Cope, PJ Lariviere, Lucio Navarro, Dennis Mishler
Current Researchers: Zuberi Ashraf, Kadena Cope, PJ Lariviere, Lucio Navarro, Dennis Mishler
![]() News: Bacteria Engineered to Protect Bees from Pests and Pathogens
News: Bacteria Engineered to Protect Bees from Pests and Pathogens
![]() Representative Publications
Representative Publications
- Lariviere et al. (2023) Nature Protocols PMID: 36460809
- Leonard et al. (2020) Science PMID: 32001655
- Leonard et al. (2018) ACS Synthetic Biology PMID: 29608282
Aphids: Understanding the Evolution of Endosymbionts
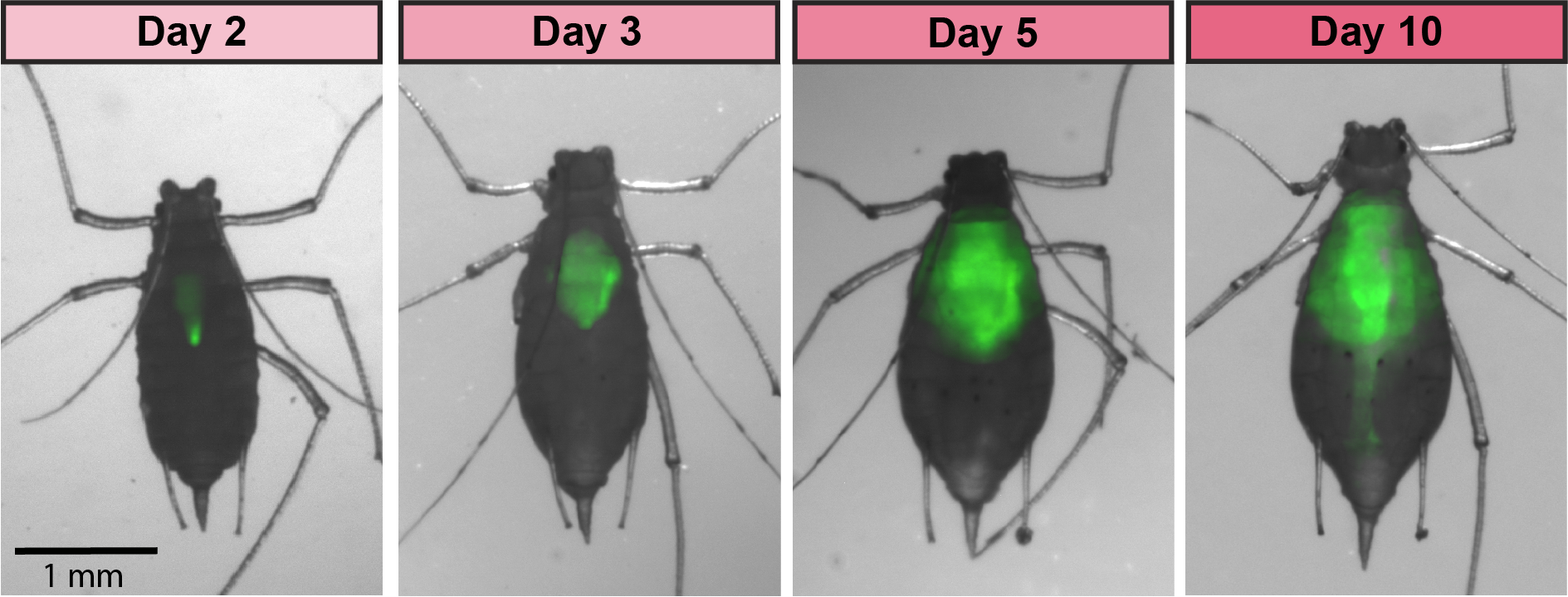
GFP-tagged symbiont colonizing the aphid gut
Aphids are plant pests and model systems for studying bacteria-insect symbioses. We have developed tools for engineering strains of Serratia symbiotica that colonize the aphid gut. We are now using these tools to unravel how these strains, which can be pathogenic to their hosts, are related to strains of S. symbiotica that live within insect cells, are inherited across aphid generations, and can benefit their aphid hosts. We have showed that cultured S. symbiotica strains are capable of maternal transmission within aphids, suggesting that they possess a latent capacity to evolve a long-term symbiotic relationship with their hosts. We are also exploring applications of these engineered bacteria related to pest control.
![]() Current Researchers: Anthony VanDieren
Current Researchers: Anthony VanDieren
![]() News: Turning Plant Pests into Helpers
News: Turning Plant Pests into Helpers
![]() Representative Publications
Representative Publications
- Elston et al. (2023) PeerJ PMID: 36874963
- Elston et al. (2020) Applied and Environmental Microbiology PMID: 33277267
- Perreau et al. (2020) mBio PMID: 35012344
Leafhoppers: Evolution and Biochemistry of Natural Nanoparticles
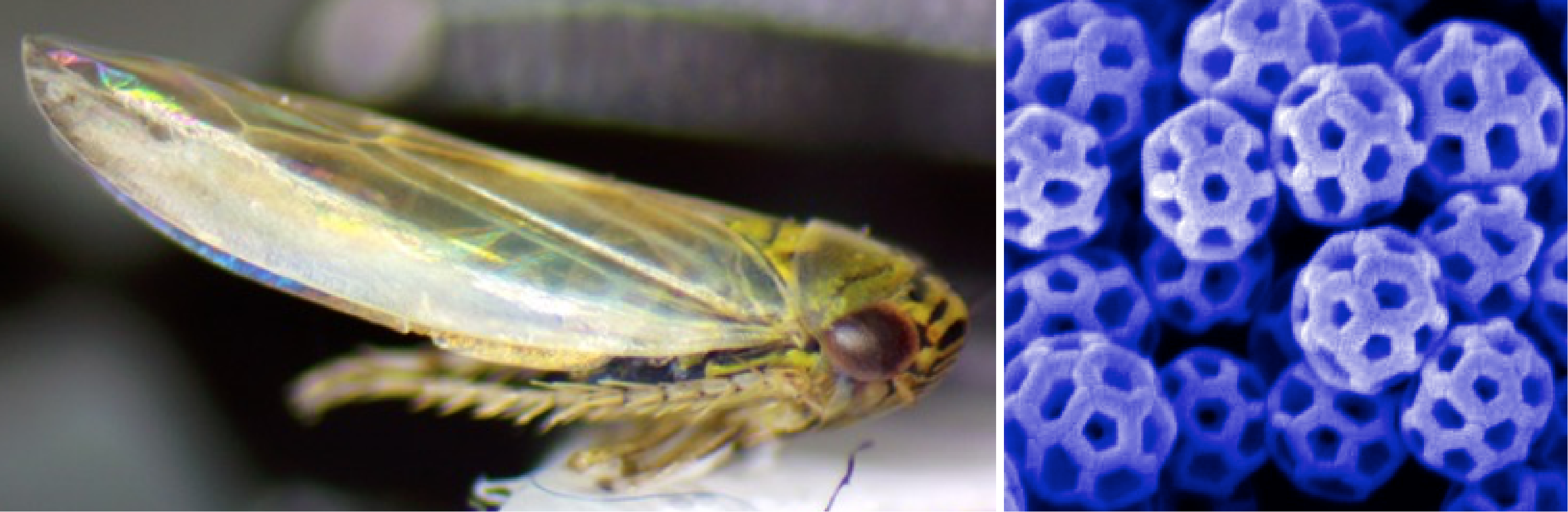
Leafhopper and SEM image of brochosomes on its wing
![]() Researchers: Jack Dwenger, Jace Gertz
Researchers: Jack Dwenger, Jace Gertz
![]() News: Tiny Insects Provide Inspiration for New Biomaterials
News: Tiny Insects Provide Inspiration for New Biomaterials
Improving the Reliability of Synthetic Biology
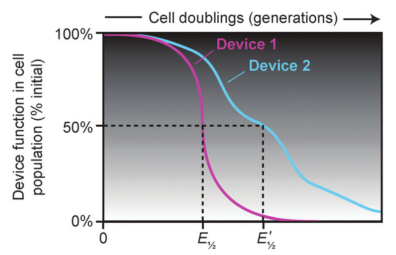
Evolutionary half-lives of biological devices
Synthetic biology applies engineering principles to create living systems with predictable and useful behaviors from collections of standardized genetic parts. However, living systems – unlike mechanical devices – inevitably evolve when their DNA sequences accumulate copying errors, often resulting in "broken" cells that no longer function as they were programmed. We are addressing this challenge by better characterizing how engineered cells evolve and using this information to design DNA sequences and host cells that are more robust against unwanted evolution. Also, precisely defining the function, extent, and provenance of genetic parts can be complicated because variation in these parts can arise from engineering or intentional evolution. This work includes: (1) developing "negative design" software to alert researchers to genetically unstable and unintentionally burdensome DNA designs; (2) using experimental evolution and engineering to create "antimutator" variants of host organisms that have lower-than-natural mutation rates; and (3) developing databases and software tools for improved annotation of engineered DNA sequences and common variants of those sequences.
![]() Current Researchers: Cameron Roots
Current Researchers: Cameron Roots
![]() Representative Publications
Representative Publications
- McGuffie & Barrick (2021) Nucleic Acids Research PMID: 34019636
- Deatherage et al. (2018) Nucleic Acids Research PMID: 30137492
- Jack et al. (2015) ACS Synthetic Biology PMID:26096262
- Renda et al. (2014) Molecular Biosystems PMID:24556867
![]() Current Funding: NIH R01; Past Funding: NSF CAREER, DARPA BRICS, DARPA Insect Allies
Current Funding: NIH R01; Past Funding: NSF CAREER, DARPA BRICS, DARPA Insect Allies
Dynamics of Microbial Genome Evolution
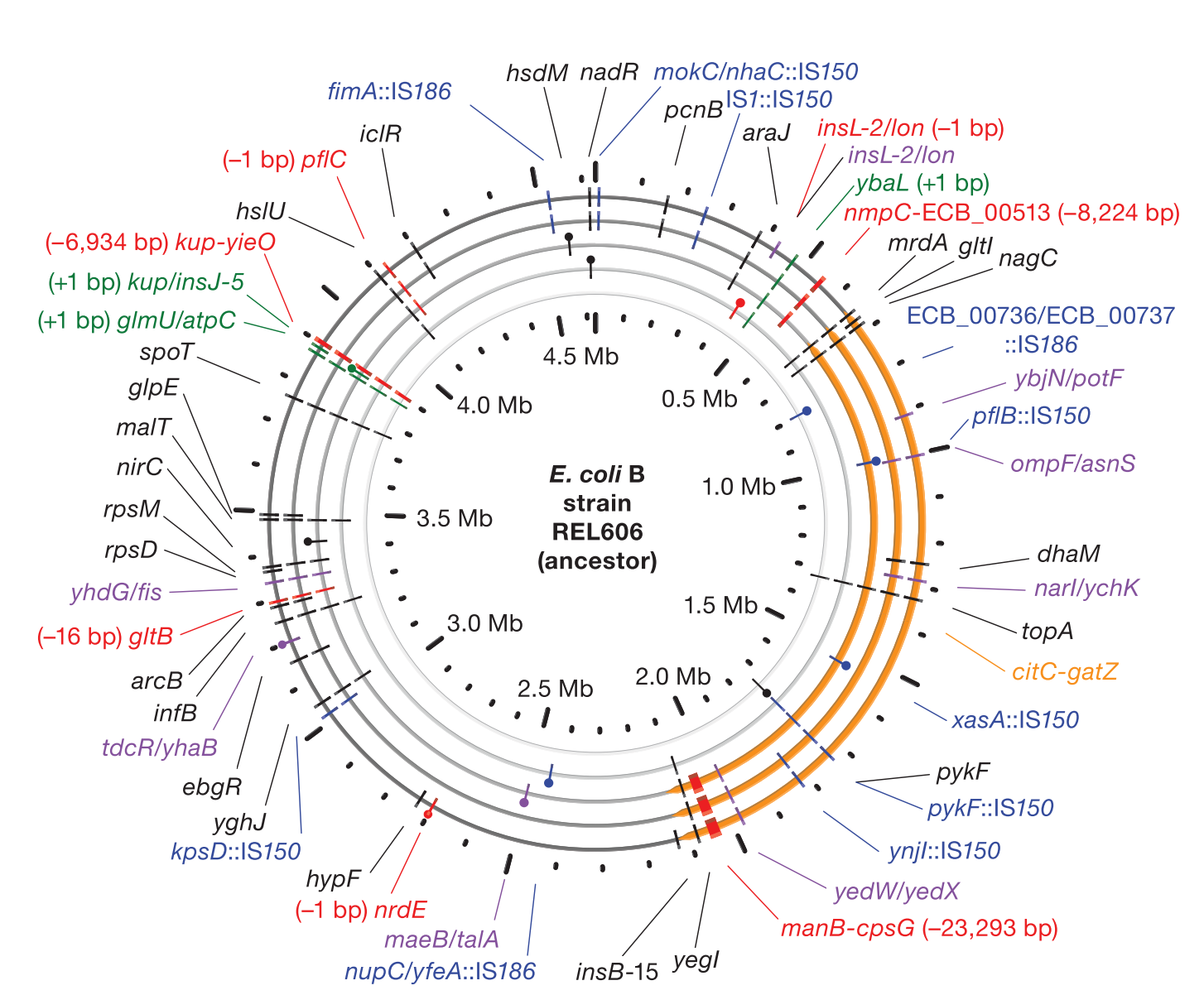
Accumulation of mutations in population Ara-1 of the LTEE over 20,000 generations of evolution
![]() Current Researchers: Daniel Deatherage, Ira Zibbu, Jack Dwenger
Current Researchers: Daniel Deatherage, Ira Zibbu, Jack Dwenger
![]() News: Legendary bacterial evolution experiment enters new era
News: Legendary bacterial evolution experiment enters new era
![]() Representative Publications
Representative Publications
- Deatherage & Barrick et al. (2021) Cell Systems PMID: 34536379
- Zhang et al. (2019) Nature Communications PMID: 31863068
- Deatherage et al. (2017) PNAS PMID: 28202733
- Deatherage and Barrick (2014) Methods Mol in Molecular Biology PMID:24838886
- Barrick and Lenski (2013) Nature Reviews Genetics. PMID:24166031
![]() Funding: NSF LTREB, UT-CNS SPARK, NSF EEID, NASA Past Funding: NSF K99/R00
Funding: NSF LTREB, UT-CNS SPARK, NSF EEID, NASA Past Funding: NSF K99/R00
Evolution and Engineering of Naturally Transformable Bacteria
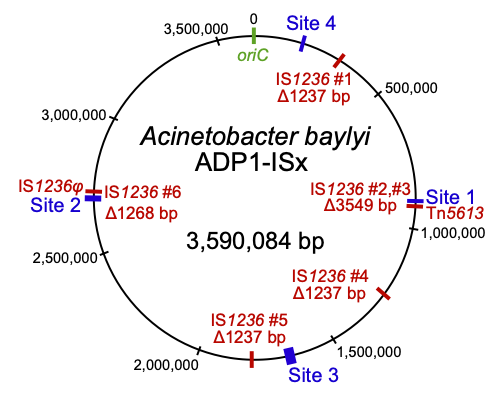
Insertion sequences (red) deleted in the transposon-free strain Acinetobacter baylyi ADP1-ISx
Naturally competent bacteria have expanded evolutionary potential because they can readily acquire new DNA from their environment. We are using experimental evolution of the model organism Acinetobacter baylyi ADP1 to understand how horizontally acquired genes and mobile genetic elements become domesticated after their incorporation into a new genome and the broader effects of gene acquisition on the rest of the genome. We are also studying the role of chemical specificity in determining the fate of acquired DNA as either nutrition or genetic information. These bacteria also provide an improved platform for studying microbial genome engineering due to the ease of reconstructing mutations and introducing new genes. We are investigating sources of genetic instability in ADP1 and engineering a clean genome version of this strain by deleting transposable elements and prophages. This will promote the use of ADP1 in synthetic biology by reducing rates of mutations that lead to inactivation of introduced genes. Further, we are using ADP1 as a platform to understand the limits to streamlining bacterial genomes and identifying adaptations to overcome the fitness costs of reduced genomes.
![]() Current Researchers: Isaac Gifford, Elizabeth Manriquez
Current Researchers: Isaac Gifford, Elizabeth Manriquez
![]() Representative Publications
Representative Publications
- Suárez et al. (2020) Nucleic Acids Research PMID:32232367
- Suárez et al. (2017) Applied and Environmental Microbiology PMID:28667117
- Renda et al. (2015) Journal of Bacteriology PMID:25512307
![]() Funding: NSF, UT-CNS SPARK Past Funding: Welch Foundation, NSF CAREER
Funding: NSF, UT-CNS SPARK Past Funding: Welch Foundation, NSF CAREER
Evolution and Engineering of Bacteriophages
The rise of multiple drug resistant pathogenic bacteria has raised the question of available, robust alternatives to antibiotics for disease treatment. Our lab is exploring new techniques for modifying bacteriophages for use in phage therapy. Evolution, engineering, and expansion of bacteriophage genomes has the potential to diversify target diseases for treatment, perform diagnostics for proactive disease prevention, and improve the efficacy of existing applications. We are working to accomplish these advancements through computational simulations and non-standard amino acid integration. The introduction of non-standard amino acids into the repertoire of protein production also brings insights into evolutionary outcomes that may not be possible in existing natural environments. Learning about evolution enabled by non-standard amino acids can lead to applications beyond the scope of viruses. We collaborate with the Wilke lab to use their Pinetree software for simulating phage gene expression and evolution.
![]() Current Researchers: Cameron Roots, Victor Li
Current Researchers: Cameron Roots, Victor Li
![]() Representative Publications
Representative Publications
- Monk et al. (2017) ACS Synthetic Biology PMID: 27648665
- Hammerling et al. (2014) Nature Chemical Biology PMID: 24838886
![]() Funding: NIH R01 Past Funding: Welch Foundation
Funding: NIH R01 Past Funding: Welch Foundation
Barrick Lab > ResearchInterests

 Mol Biosciences
Mol Biosciences The LTEE
The LTEE iGEM team
iGEM team NGS course
NGS course