| |
| META TOPICPARENT |
name="QPCR" |
<<Return to qPCR page
Reference Gene qPCR
Goals
- Determine which of your reference genes you are going to normalize to.
Why am I doing this? |
|
<
< | Reference genes need to be stable across your control and experimental samples in order to be useful. If expression between the two differs, you will be normalizing to two different values, reference genes should always be validated before you use them in your experiments! |
>
> | Reference genes need to be stable across your control and experimental samples in order to be useful. If expression between the two differs, you will be normalizing to two different values. |
|
>
> | Reference genes should always be validated before you use them in your experiments. |
| |
Typical plate setup for three candidate reference genes:
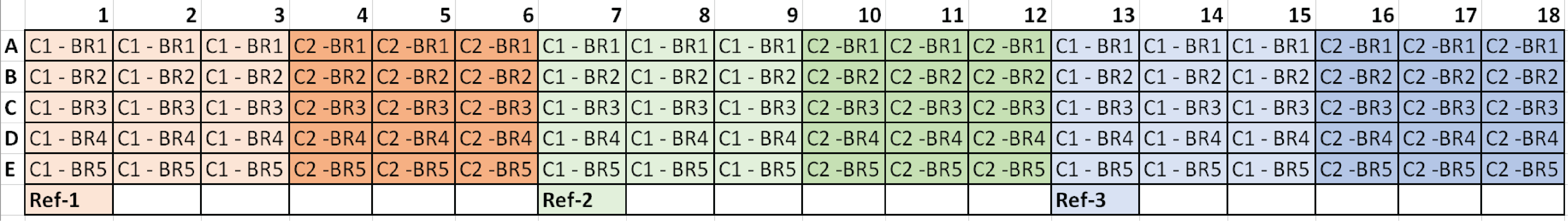 *C1 = Condition 1, C2 = Condition 2, and BR# = Biological Replicate
*C1 = Condition 1, C2 = Condition 2, and BR# = Biological Replicate |
|
<
< | Template Material_ |
>
> | Template Material |
| |
What you are looking for |
|
<
< |
- Technical replicates with a standard deviation below 0.2 (this confirms the accuracy of your results).
- At least 2 reference primer sets that show no significant difference between control and experimental conditions. The standard deviation of the Cqs for all biological replicates should be low. (Preferrably Less than 0.5)
- If you are seeing differences between replicates or perhaps conditions, the main issue is likely from setting up the qPCR plate itself. The first few times you run qPCR the results can be messy and sometimes you just have to redo things to get accurate results. That being said, if you're a qPCR pro, or if you keep getting the same results over and over you may have to design primers for a new reference.
|
>
> |
- Technical replicates with a standard deviation below 0.2. This confirms the accuracy of your results.
- At least 2 reference primer sets that show no significant difference between control and experimental conditions. The standard deviation of the Cqs for all biological replicates should be low, preferrably less than 0.5.
- If you are seeing differences between replicates and conditions, it is likely due to technical issues encountered when setting up the qPCR plate. The first few times you run qPCR the results can be messy, and sometimes you have to redo things to get accurate results. That being said, if you're a qPCR pro, or if you keep getting the same results over and over you may have to design primers for a new reference.
|
| |
<<Return to qPCR page
| META FILEATTACHMENT |
attachment="Refprimerplate.png" attr="h" comment="" date="1582235087" name="Refprimerplate.png" path="Refprimerplate.png" size="102909" stream="Refprimerplate.png" tmpFilename="/usr/tmp/CGItemp43424" user="KateElston" version="2" |
|

 *C1 = Condition 1, C2 = Condition 2, and BR# = Biological Replicate
*C1 = Condition 1, C2 = Condition 2, and BR# = Biological Replicate