
Difference: GoldenGateAssemblyProtocolsMainPage (1 vs. 16)
Revision 162025-06-17 - AlexaMorton
Golden Gate AssemblyThis page serves as the main repository for everything Golden Gate Assembly-related. This page links to a number of protocol pages that will help you design DNA sequences for Golden Gate Assembly, learn about the various different Golden Gate Assembly techniques and when we employ each one, and conduct the different types of Golden Gate Assembly reactions. Background & Design:Golden Gate Assembly (GGA) was first described in Engler C, Kandzia R, Marillonnet S (2008) and Engler C, Gruetzner R, Kandzia R, Marillonnet S (2009) as an efficient way to quickly assemble multiple DNA sequences, or parts, into a single plasmid. This molecular cloning method allows a researcher to simultaneously and directionally assemble multiple DNA fragments into a single piece using Type IIs restriction enzymes and T4/T7 DNA ligase. This assembly is performed in vitro. The most commonly used Type IIs enzymes are BsaI, BsmBI and BbsI. Unlike standard Type II restriction enzymes like EcoRI and BamHI, these enzymes cut DNA outside of their recognition sites and therefore can create customized overhangs. Since 256 potential overhang sequences are possible, multiple fragments of DNA can be assembled by using combinations of overhang sequences. Golden Gate Assembly LinksGeneral Golden Gate Assembly Protocols
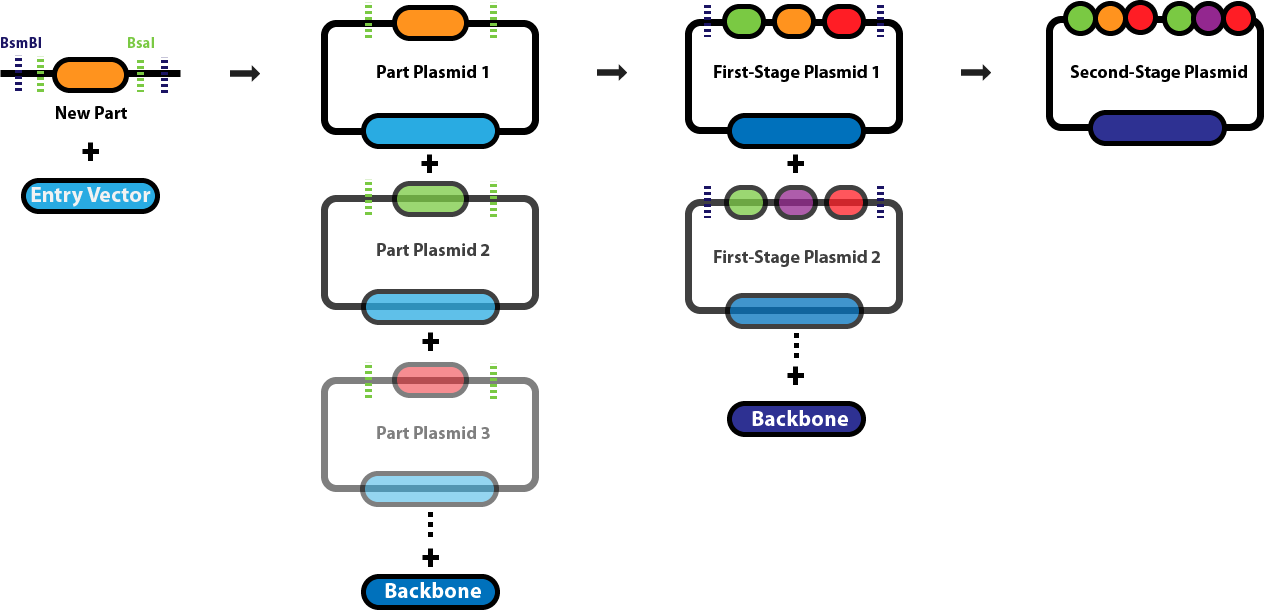
| |||||||||||||||
| Added: | |||||||||||||||
| > > |
| ||||||||||||||
iGEM-Specific Protocols
References
Contributors
-- Main.KateElston - 29 Jan 2018 The excel worksheet for calculating Golden Gate Assembly Reactions can be found in the attachment below.
| |||||||||||||||
Revision 152021-11-03 - KateElston
Golden Gate AssemblyThis page serves as the main repository for everything Golden Gate Assembly-related. This page links to a number of protocol pages that will help you design DNA sequences for Golden Gate Assembly, learn about the various different Golden Gate Assembly techniques and when we employ each one, and conduct the different types of Golden Gate Assembly reactions. Background & Design:Golden Gate Assembly (GGA) was first described in Engler C, Kandzia R, Marillonnet S (2008) and Engler C, Gruetzner R, Kandzia R, Marillonnet S (2009) as an efficient way to quickly assemble multiple DNA sequences, or parts, into a single plasmid. This molecular cloning method allows a researcher to simultaneously and directionally assemble multiple DNA fragments into a single piece using Type IIs restriction enzymes and T4/T7 DNA ligase. This assembly is performed in vitro. The most commonly used Type IIs enzymes are BsaI, BsmBI and BbsI. Unlike standard Type II restriction enzymes like EcoRI and BamHI, these enzymes cut DNA outside of their recognition sites and therefore can create customized overhangs. Since 256 potential overhang sequences are possible, multiple fragments of DNA can be assembled by using combinations of overhang sequences. | |||||||||||
| Changed: | |||||||||||
| < < | Golden Gate Assembly Schematic: | ||||||||||
| > > | Golden Gate Assembly Links | ||||||||||
| Changed: | |||||||||||
| < < |  | ||||||||||
| > > | General Golden Gate Assembly Protocols | ||||||||||
| Added: | |||||||||||
| > > |
| ||||||||||
| Changed: | |||||||||||
| < < | Golden Gate Assembly Links | ||||||||||
| > > | Bee Toolkit Golden Gate Assembly Scheme- Start to Finish | ||||||||||
| Added: | |||||||||||
| > > |
 | ||||||||||
| Deleted: | |||||||||||
| < < | Golden Gate Assembly - Start to Finish | ||||||||||
| |||||||||||
| Deleted: | |||||||||||
| < < |
| ||||||||||
Useful Tools
| |||||||||||
| Changed: | |||||||||||
| < < |
| ||||||||||
| > > |
| ||||||||||
References
Contributors
-- Main.KateElston - 29 Jan 2018 The excel worksheet for calculating Golden Gate Assembly Reactions can be found in the attachment below. | |||||||||||
| Changed: | |||||||||||
| < < |
| ||||||||||
| > > |
| ||||||||||
| |||||||||||
| Added: | |||||||||||
| > > |
| ||||||||||
Revision 142021-07-12 - KateElston
Golden Gate AssemblyThis page serves as the main repository for everything Golden Gate Assembly-related. This page links to a number of protocol pages that will help you design DNA sequences for Golden Gate Assembly, learn about the various different Golden Gate Assembly techniques and when we employ each one, and conduct the different types of Golden Gate Assembly reactions. Background & Design:Golden Gate Assembly (GGA) was first described in Engler C, Kandzia R, Marillonnet S (2008) and Engler C, Gruetzner R, Kandzia R, Marillonnet S (2009) as an efficient way to quickly assemble multiple DNA sequences, or parts, into a single plasmid. This molecular cloning method allows a researcher to simultaneously and directionally assemble multiple DNA fragments into a single piece using Type IIs restriction enzymes and T4/T7 DNA ligase. This assembly is performed in vitro. The most commonly used Type IIs enzymes are BsaI, BsmBI and BbsI. Unlike standard Type II restriction enzymes like EcoRI and BamHI, these enzymes cut DNA outside of their recognition sites and therefore can create customized overhangs. Since 256 potential overhang sequences are possible, multiple fragments of DNA can be assembled by using combinations of overhang sequences. Golden Gate Assembly Schematic: 
Golden Gate Assembly LinksGolden Gate Assembly - Start to Finish
| |||||||||||||
| Deleted: | |||||||||||||
| < < |
Kit-Specific Pages
| ||||||||||||
iGEM-Specific Protocols
References
Contributors
-- Main.KateElston - 29 Jan 2018 The excel worksheet for calculating Golden Gate Assembly Reactions can be found in the attachment below.
| |||||||||||||
Revision 132021-07-09 - KateElston
Golden Gate AssemblyThis page serves as the main repository for everything Golden Gate Assembly-related. This page links to a number of protocol pages that will help you design DNA sequences for Golden Gate Assembly, learn about the various different Golden Gate Assembly techniques and when we employ each one, and conduct the different types of Golden Gate Assembly reactions. Background & Design:Golden Gate Assembly (GGA) was first described in Engler C, Kandzia R, Marillonnet S (2008) and Engler C, Gruetzner R, Kandzia R, Marillonnet S (2009) as an efficient way to quickly assemble multiple DNA sequences, or parts, into a single plasmid. This molecular cloning method allows a researcher to simultaneously and directionally assemble multiple DNA fragments into a single piece using Type IIs restriction enzymes and T4/T7 DNA ligase. This assembly is performed in vitro. The most commonly used Type IIs enzymes are BsaI, BsmBI and BbsI. Unlike standard Type II restriction enzymes like EcoRI and BamHI, these enzymes cut DNA outside of their recognition sites and therefore can create customized overhangs. Since 256 potential overhang sequences are possible, multiple fragments of DNA can be assembled by using combinations of overhang sequences. Golden Gate Assembly Schematic: 
Golden Gate Assembly LinksGolden Gate Assembly - Start to Finish
| |||||||||||
| Added: | |||||||||||
| > > | Useful Tools
| ||||||||||
Kit-Specific Pages
References
Contributors
-- Main.KateElston - 29 Jan 2018 The excel worksheet for calculating Golden Gate Assembly Reactions can be found in the attachment below. | |||||||||||
| Changed: | |||||||||||
| < < |
| ||||||||||
| > > |
| ||||||||||
| |||||||||||
Revision 122021-04-16 - JeffreyBarrick
Golden Gate AssemblyThis page serves as the main repository for everything Golden Gate Assembly-related. This page links to a number of protocol pages that will help you design DNA sequences for Golden Gate Assembly, learn about the various different Golden Gate Assembly techniques and when we employ each one, and conduct the different types of Golden Gate Assembly reactions. | |||||||||||||
| Changed: | |||||||||||||
| < < | Background & Design: Golden Gate Assembly (GGA) was first described in Engler C, Kandzia R, Marillonnet S (2008) and Engler C, Gruetzner R, Kandzia R, Marillonnet S (2009) as an efficient way to quickly assembly multiple DNA sequences, or parts, into a single plasmid. This molecular cloning method allows a researcher to simultaneously and directionally assemble multiple DNA fragments into a single piece using Type IIs restriction enzymes and T4/T7 DNA ligase. This assembly is performed in vitro. The most commonly used Type IIs enzymes are BsaI, BsmBI and BbsI. Unlike standard Type II restriction enzymes like EcoRI and BamHI, these enzymes cut DNA outside of their recognition sites and therefore can create non-palindromic overhangs. Since 256 potential overhang sequences are possible, multiple fragments of DNA can be assembled by using combinations of overhang sequences. | ||||||||||||
| > > | Background & Design: Golden Gate Assembly (GGA) was first described in Engler C, Kandzia R, Marillonnet S (2008) and Engler C, Gruetzner R, Kandzia R, Marillonnet S (2009) as an efficient way to quickly assemble multiple DNA sequences, or parts, into a single plasmid. This molecular cloning method allows a researcher to simultaneously and directionally assemble multiple DNA fragments into a single piece using Type IIs restriction enzymes and T4/T7 DNA ligase. This assembly is performed in vitro. The most commonly used Type IIs enzymes are BsaI, BsmBI and BbsI. Unlike standard Type II restriction enzymes like EcoRI and BamHI, these enzymes cut DNA outside of their recognition sites and therefore can create customized overhangs. Since 256 potential overhang sequences are possible, multiple fragments of DNA can be assembled by using combinations of overhang sequences. | ||||||||||||
Golden Gate Assembly Schematic:

Golden Gate Assembly LinksGolden Gate Assembly - Start to Finish
References
Contributors
-- Main.KateElston - 29 Jan 2018 The excel worksheet for calculating Golden Gate Assembly Reactions can be found in the attachment below.
| |||||||||||||
Revision 112018-06-21 - KateElston
Golden Gate AssemblyThis page serves as the main repository for everything Golden Gate Assembly-related. This page links to a number of protocol pages that will help you design DNA sequences for Golden Gate Assembly, learn about the various different Golden Gate Assembly techniques and when we employ each one, and conduct the different types of Golden Gate Assembly reactions. Background & Design:Golden Gate Assembly (GGA) was first described in Engler C, Kandzia R, Marillonnet S (2008) and Engler C, Gruetzner R, Kandzia R, Marillonnet S (2009) as an efficient way to quickly assembly multiple DNA sequences, or parts, into a single plasmid. This molecular cloning method allows a researcher to simultaneously and directionally assemble multiple DNA fragments into a single piece using Type IIs restriction enzymes and T4/T7 DNA ligase. This assembly is performed in vitro. The most commonly used Type IIs enzymes are BsaI, BsmBI and BbsI. Unlike standard Type II restriction enzymes like EcoRI and BamHI, these enzymes cut DNA outside of their recognition sites and therefore can create non-palindromic overhangs. Since 256 potential overhang sequences are possible, multiple fragments of DNA can be assembled by using combinations of overhang sequences. Golden Gate Assembly Schematic: 
Golden Gate Assembly LinksGolden Gate Assembly - Start to Finish
| |||||||||||||
| Deleted: | |||||||||||||
| < < |
| ||||||||||||
References
Contributors
-- Main.KateElston - 29 Jan 2018 The excel worksheet for calculating Golden Gate Assembly Reactions can be found in the attachment below.
| |||||||||||||
Revision 102018-06-07 - DennisMishler
Golden Gate AssemblyThis page serves as the main repository for everything Golden Gate Assembly-related. This page links to a number of protocol pages that will help you design DNA sequences for Golden Gate Assembly, learn about the various different Golden Gate Assembly techniques and when we employ each one, and conduct the different types of Golden Gate Assembly reactions. Background & Design:Golden Gate Assembly (GGA) was first described in Engler C, Kandzia R, Marillonnet S (2008) and Engler C, Gruetzner R, Kandzia R, Marillonnet S (2009) as an efficient way to quickly assembly multiple DNA sequences, or parts, into a single plasmid. This molecular cloning method allows a researcher to simultaneously and directionally assemble multiple DNA fragments into a single piece using Type IIs restriction enzymes and T4/T7 DNA ligase. This assembly is performed in vitro. The most commonly used Type IIs enzymes are BsaI, BsmBI and BbsI. Unlike standard Type II restriction enzymes like EcoRI and BamHI, these enzymes cut DNA outside of their recognition sites and therefore can create non-palindromic overhangs. Since 256 potential overhang sequences are possible, multiple fragments of DNA can be assembled by using combinations of overhang sequences. Golden Gate Assembly Schematic: 
Golden Gate Assembly LinksGolden Gate Assembly - Start to Finish
References
Contributors
-- Main.KateElston - 29 Jan 2018 | |||||||||||||
| Added: | |||||||||||||
| > > |
The excel worksheet for calculating Golden Gate Assembly Reactions can be found in the attachment below. | ||||||||||||
| |||||||||||||
Revision 92018-03-22 - KateElston
Golden Gate AssemblyThis page serves as the main repository for everything Golden Gate Assembly-related. This page links to a number of protocol pages that will help you design DNA sequences for Golden Gate Assembly, learn about the various different Golden Gate Assembly techniques and when we employ each one, and conduct the different types of Golden Gate Assembly reactions. Background & Design:Golden Gate Assembly (GGA) was first described in Engler C, Kandzia R, Marillonnet S (2008) and Engler C, Gruetzner R, Kandzia R, Marillonnet S (2009) as an efficient way to quickly assembly multiple DNA sequences, or parts, into a single plasmid. This molecular cloning method allows a researcher to simultaneously and directionally assemble multiple DNA fragments into a single piece using Type IIs restriction enzymes and T4/T7 DNA ligase. This assembly is performed in vitro. The most commonly used Type IIs enzymes are BsaI, BsmBI and BbsI. Unlike standard Type II restriction enzymes like EcoRI and BamHI, these enzymes cut DNA outside of their recognition sites and therefore can create non-palindromic overhangs. Since 256 potential overhang sequences are possible, multiple fragments of DNA can be assembled by using combinations of overhang sequences. Golden Gate Assembly Schematic: | ||||||||
| Changed: | ||||||||
| < < | 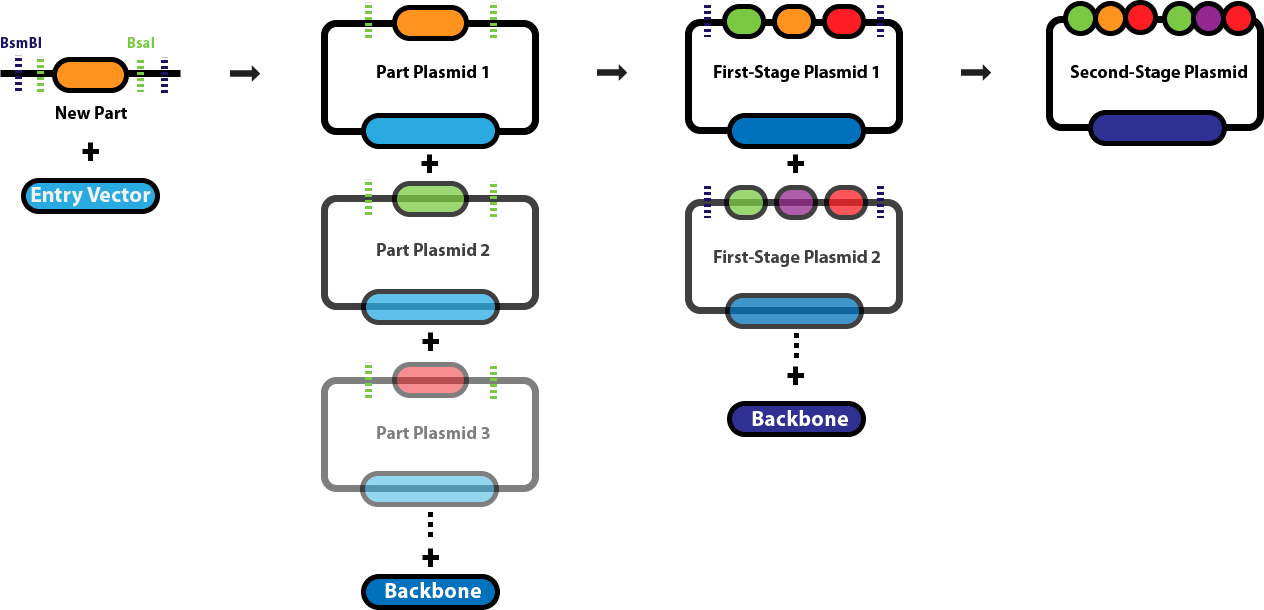 | |||||||
| > > |  | |||||||
Golden Gate Assembly LinksGolden Gate Assembly - Start to Finish
References
Contributors
-- Main.KateElston - 29 Jan 2018
| ||||||||
| Changed: | ||||||||
| < < |
| |||||||
| > > |
| |||||||
| ||||||||
| Changed: | ||||||||
| < < |
| |||||||
| > > |
| |||||||
| Added: | ||||||||
| > > |
| |||||||
Revision 82018-03-22 - SeanLeonard
Golden Gate AssemblyThis page serves as the main repository for everything Golden Gate Assembly-related. This page links to a number of protocol pages that will help you design DNA sequences for Golden Gate Assembly, learn about the various different Golden Gate Assembly techniques and when we employ each one, and conduct the different types of Golden Gate Assembly reactions. | |||||||||||
| Changed: | |||||||||||
| < < | Background & Design: Golden Gate Assembly (GGA) was first described in Engler C, Kandzia R, Marillonnet S (2008) and Engler C, Gruetzner R, Kandzia R, Marillonnet S (2009) as an efficient way to quickly assembly multiple DNA sequences, or parts, into a single plasmid. This molecular cloning method allows a researcher to simultaneously and directionally assemble multiple DNA fragments into a single piece using Type IIs restriction enzymes and T4/T7 DNA ligase. This assembly is performed in vitro. Most commonly used Type IIs enzymes include BsaI, BsmBI and BbsI. Unlike standard Type II restriction enzymes like EcoRI and BamHI, these enzymes cut DNA outside of their recognition sites and therefore can create non-palindromic overhangs. Since 256 potential overhang sequences are possible, multiple fragments of DNA can be assembled by using combinations of overhang sequences. | ||||||||||
| > > | Background & Design: Golden Gate Assembly (GGA) was first described in Engler C, Kandzia R, Marillonnet S (2008) and Engler C, Gruetzner R, Kandzia R, Marillonnet S (2009) as an efficient way to quickly assembly multiple DNA sequences, or parts, into a single plasmid. This molecular cloning method allows a researcher to simultaneously and directionally assemble multiple DNA fragments into a single piece using Type IIs restriction enzymes and T4/T7 DNA ligase. This assembly is performed in vitro. The most commonly used Type IIs enzymes are BsaI, BsmBI and BbsI. Unlike standard Type II restriction enzymes like EcoRI and BamHI, these enzymes cut DNA outside of their recognition sites and therefore can create non-palindromic overhangs. Since 256 potential overhang sequences are possible, multiple fragments of DNA can be assembled by using combinations of overhang sequences. | ||||||||||
| Golden Gate Assembly Schematic: | |||||||||||
| Changed: | |||||||||||
| < < |  | ||||||||||
| > > |  | ||||||||||
Golden Gate Assembly LinksGolden Gate Assembly - Start to Finish | |||||||||||
| Changed: | |||||||||||
| < < |
| ||||||||||
| > > |
| ||||||||||
References
Contributors
-- Main.KateElston - 29 Jan 2018
| |||||||||||
Revision 72018-03-22 - KateElston
Golden Gate AssemblyThis page serves as the main repository for everything Golden Gate Assembly-related. This page links to a number of protocol pages that will help you design DNA sequences for Golden Gate Assembly, learn about the various different Golden Gate Assembly techniques and when we employ each one, and conduct the different types of Golden Gate Assembly reactions. Background & Design:Golden Gate Assembly (GGA) was first described in Engler C, Kandzia R, Marillonnet S (2008) and Engler C, Gruetzner R, Kandzia R, Marillonnet S (2009) as an efficient way to quickly assembly multiple DNA sequences, or parts, into a single plasmid. This molecular cloning method allows a researcher to simultaneously and directionally assemble multiple DNA fragments into a single piece using Type IIs restriction enzymes and T4/T7 DNA ligase. This assembly is performed in vitro. Most commonly used Type IIs enzymes include BsaI, BsmBI and BbsI. Unlike standard Type II restriction enzymes like EcoRI and BamHI, these enzymes cut DNA outside of their recognition sites and therefore can create non-palindromic overhangs. Since 256 potential overhang sequences are possible, multiple fragments of DNA can be assembled by using combinations of overhang sequences. Golden Gate Assembly Schematic: 
Golden Gate Assembly LinksGolden Gate Assembly - Start to Finish
References
Contributors
-- Main.KateElston - 29 Jan 2018 | |||||||||
| Changed: | |||||||||
| < < |
| ||||||||
| > > |
| ||||||||
| |||||||||
Revision 62018-01-31 - KateElston
Golden Gate AssemblyThis page serves as the main repository for everything Golden Gate Assembly-related. This page links to a number of protocol pages that will help you design DNA sequences for Golden Gate Assembly, learn about the various different Golden Gate Assembly techniques and when we employ each one, and conduct the different types of Golden Gate Assembly reactions. Background & Design:Golden Gate Assembly (GGA) was first described in Engler C, Kandzia R, Marillonnet S (2008) and Engler C, Gruetzner R, Kandzia R, Marillonnet S (2009) as an efficient way to quickly assembly multiple DNA sequences, or parts, into a single plasmid. This molecular cloning method allows a researcher to simultaneously and directionally assemble multiple DNA fragments into a single piece using Type IIs restriction enzymes and T4/T7 DNA ligase. This assembly is performed in vitro. Most commonly used Type IIs enzymes include BsaI, BsmBI and BbsI. Unlike standard Type II restriction enzymes like EcoRI and BamHI, these enzymes cut DNA outside of their recognition sites and therefore can create non-palindromic overhangs. Since 256 potential overhang sequences are possible, multiple fragments of DNA can be assembled by using combinations of overhang sequences. Golden Gate Assembly Schematic: 
Golden Gate Assembly LinksGolden Gate Assembly - Start to Finish
References
Contributors
-- Main.KateElston - 29 Jan 2018 | |||||||||
| Changed: | |||||||||
| < < |
| ||||||||
| > > |
| ||||||||
| |||||||||
Revision 52018-01-31 - JuliePerreau
Golden Gate Assembly | |||||||||||
| Changed: | |||||||||||
| < < | This page serves as the main repository for everything Golden Gate Assembly-related. This page links to a number of protocol pages that will help you design DNA sequences for Golden Gate Assembly, learn about the various different Golden Gate Assembly techniques and when we employ each one, and conduct the different types of Golden Gate Assembly reactions. | ||||||||||
| > > | This page serves as the main repository for everything Golden Gate Assembly-related. This page links to a number of protocol pages that will help you design DNA sequences for Golden Gate Assembly, learn about the various different Golden Gate Assembly techniques and when we employ each one, and conduct the different types of Golden Gate Assembly reactions. | ||||||||||
| Changed: | |||||||||||
| < < | Background & Design: | ||||||||||
| > > | Background & Design: Golden Gate Assembly (GGA) was first described in Engler C, Kandzia R, Marillonnet S (2008) and Engler C, Gruetzner R, Kandzia R, Marillonnet S (2009) as an efficient way to quickly assembly multiple DNA sequences, or parts, into a single plasmid. This molecular cloning method allows a researcher to simultaneously and directionally assemble multiple DNA fragments into a single piece using Type IIs restriction enzymes and T4/T7 DNA ligase. This assembly is performed in vitro. Most commonly used Type IIs enzymes include BsaI, BsmBI and BbsI. Unlike standard Type II restriction enzymes like EcoRI and BamHI, these enzymes cut DNA outside of their recognition sites and therefore can create non-palindromic overhangs. Since 256 potential overhang sequences are possible, multiple fragments of DNA can be assembled by using combinations of overhang sequences. | ||||||||||
| Deleted: | |||||||||||
| < < | Golden Gate Assembly (GGA) was first described in Engler C, Kandzia R, Marillonnet S (2008) and Engler C, Gruetzner R, Kandzia R, Marillonnet S (2009) as an efficient way to quickly assembly multiple DNA sequences, or parts, into a single plasmid. This molecular cloning method allows a researcher to simultaneously and directionally assemble multiple DNA fragments into a single piece using Type IIs restriction enzymes and T4/T7 DNA ligase. This assembly is performed in vitro. Most commonly used Type IIs enzymes include BsaI, BsmBI and BbsI. Unlike standard Type II restriction enzymes like EcoRI and BamHI, these enzymes cut DNA outside of their recognition sites and therefore can create non-palindromic overhangs. Since 256 potential overhang sequences are possible, multiple fragments of DNA can be assembled by using combinations of overhang sequences. | ||||||||||
| Changed: | |||||||||||
| < < | Golden Gate Assembly Schematic: | ||||||||||
| > > | Golden Gate Assembly Schematic: | ||||||||||
| Deleted: | |||||||||||
| < < | 
| ||||||||||
| Added: | |||||||||||
| > > |  | ||||||||||
Golden Gate Assembly LinksGolden Gate Assembly - Start to Finish
| |||||||||||
| Deleted: | |||||||||||
| < < | |||||||||||
Kit-Specific Pages
| |||||||||||
| Deleted: | |||||||||||
| < < | Supplies
ProtocolUse the Calculator Spreadsheet (linked at the bottom of the page) for planning your reactions!
| ||||||||||
References | |||||||||||
| Changed: | |||||||||||
| < < |
| ||||||||||
| > > |
| ||||||||||
| Added: | |||||||||||
| > > |
| ||||||||||
Contributors
| |||||||||||
| Changed: | |||||||||||
| < < | -- Main.KateElston - 29 Jan 2018 | ||||||||||
| > > | -- Main.KateElston - 29 Jan 2018 | ||||||||||
| Deleted: | |||||||||||
| < < | |||||||||||
| |||||||||||
Revision 42018-01-30 - KateElston
Golden Gate AssemblyThis page serves as the main repository for everything Golden Gate Assembly-related. This page links to a number of protocol pages that will help you design DNA sequences for Golden Gate Assembly, learn about the various different Golden Gate Assembly techniques and when we employ each one, and conduct the different types of Golden Gate Assembly reactions. Background & Design:Golden Gate Assembly (GGA) was first described in Engler C, Kandzia R, Marillonnet S (2008) and Engler C, Gruetzner R, Kandzia R, Marillonnet S (2009) as an efficient way to quickly assembly multiple DNA sequences, or parts, into a single plasmid. This molecular cloning method allows a researcher to simultaneously and directionally assemble multiple DNA fragments into a single piece using Type IIs restriction enzymes and T4/T7 DNA ligase. This assembly is performed in vitro. Most commonly used Type IIs enzymes include BsaI, BsmBI and BbsI. Unlike standard Type II restriction enzymes like EcoRI and BamHI, these enzymes cut DNA outside of their recognition sites and therefore can create non-palindromic overhangs. Since 256 potential overhang sequences are possible, multiple fragments of DNA can be assembled by using combinations of overhang sequences. Golden Gate Assembly Schematic: | ||||||||
| Changed: | ||||||||
| < < | 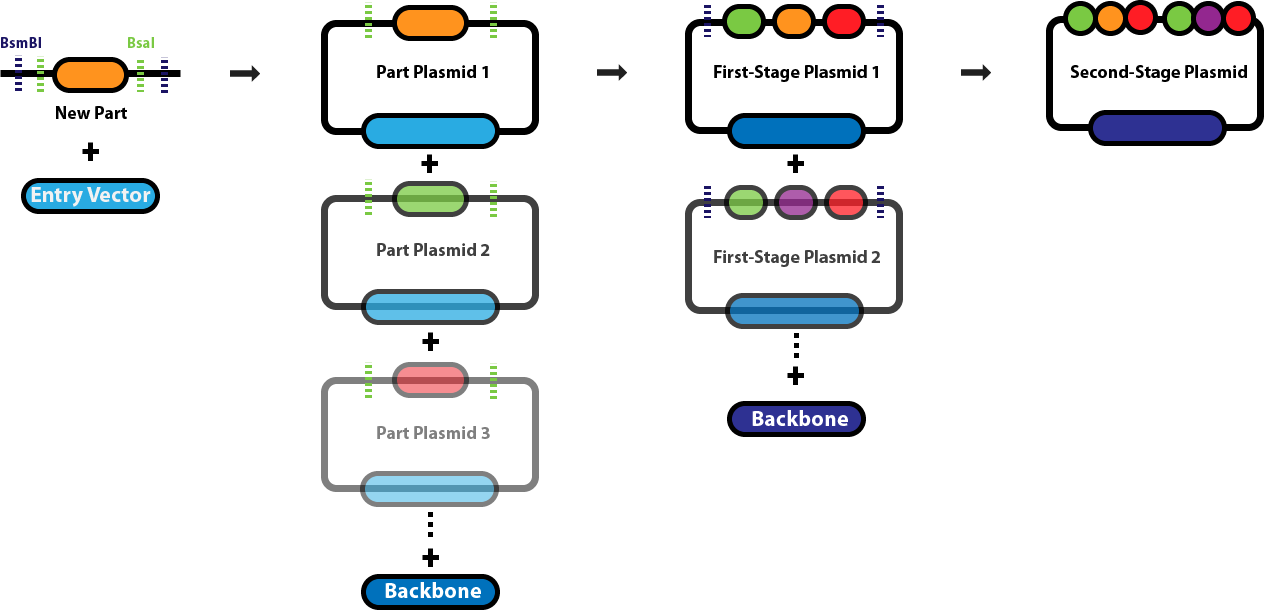 | |||||||
| > > | ||||||||
| Added: | ||||||||
| > > | 
| |||||||
| Added: | ||||||||
| > > | Golden Gate Assembly Links | |||||||
| Added: | ||||||||
| > > | Golden Gate Assembly - Start to Finish
| |||||||
Supplies
ProtocolUse the Calculator Spreadsheet (linked at the bottom of the page) for planning your reactions!
| ||||||||
| Changed: | ||||||||
| < < | Golden Gate Assembly | |||||||
| > > | References | |||||||
| Deleted: | ||||||||
| < < | Golden Gate Assembly - Start to Finish
References | |||||||
| ||||||||
| Changed: | ||||||||
| < < | Contributors | |||||||
| > > | Contributors | |||||||
-- Main.KateElston - 29 Jan 2018 | ||||||||
| Added: | ||||||||
| > > | ||||||||
| ||||||||
| Added: | ||||||||
| > > |
| |||||||
Revision 32018-01-30 - KateElston
Golden Gate AssemblyThis page serves as the main repository for everything Golden Gate Assembly-related. This page links to a number of protocol pages that will help you design DNA sequences for Golden Gate Assembly, learn about the various different Golden Gate Assembly techniques and when we employ each one, and conduct the different types of Golden Gate Assembly reactions. | ||||||||
| Changed: | ||||||||
| < < | Background & Design | |||||||
| > > | Background & Design: | |||||||
| Golden Gate Assembly (GGA) was first described in Engler C, Kandzia R, Marillonnet S (2008) and Engler C, Gruetzner R, Kandzia R, Marillonnet S (2009) as an efficient way to quickly assembly multiple DNA sequences, or parts, into a single plasmid. This molecular cloning method allows a researcher to simultaneously and directionally assemble multiple DNA fragments into a single piece using Type IIs restriction enzymes and T4/T7 DNA ligase. This assembly is performed in vitro. Most commonly used Type IIs enzymes include BsaI, BsmBI and BbsI. Unlike standard Type II restriction enzymes like EcoRI and BamHI, these enzymes cut DNA outside of their recognition sites and therefore can create non-palindromic overhangs. Since 256 potential overhang sequences are possible, multiple fragments of DNA can be assembled by using combinations of overhang sequences. | ||||||||
| Added: | ||||||||
| > > | Golden Gate Assembly Schematic:  | |||||||
Supplies
ProtocolUse the Calculator Spreadsheet (linked at the bottom of the page) for planning your reactions!
Golden Gate AssemblyGolden Gate Assembly - Start to Finish
References
Contributors
-- Main.KateElston - 29 Jan 2018
| ||||||||
| Added: | ||||||||
| > > |
| |||||||
Revision 22018-01-29 - KateElston
| ||||||||
| Changed: | ||||||||
| < < | -- Main.DennisMishler - 14 Dec 2017 | |||||||
| > > | Golden Gate Assembly | |||||||
| Deleted: | ||||||||
| < < | ||||||||
| This page serves as the main repository for everything Golden Gate Assembly-related. This page links to a number of protocol pages that will help you design DNA sequences for Golden Gate Assembly, learn about the various different Golden Gate Assembly techniques and when we employ each one, and conduct the different types of Golden Gate Assembly reactions. | ||||||||
| Added: | ||||||||
| > > | Background & Design | |||||||
| Golden Gate Assembly (GGA) was first described in Engler C, Kandzia R, Marillonnet S (2008) and Engler C, Gruetzner R, Kandzia R, Marillonnet S (2009) as an efficient way to quickly assembly multiple DNA sequences, or parts, into a single plasmid. | ||||||||
| Added: | ||||||||
| > > | This molecular cloning method allows a researcher to simultaneously and directionally assemble multiple DNA fragments into a single piece using Type IIs restriction enzymes and T4/T7 DNA ligase. This assembly is performed in vitro. Most commonly used Type IIs enzymes include BsaI, BsmBI and BbsI. Unlike standard Type II restriction enzymes like EcoRI and BamHI, these enzymes cut DNA outside of their recognition sites and therefore can create non-palindromic overhangs. Since 256 potential overhang sequences are possible, multiple fragments of DNA can be assembled by using combinations of overhang sequences. | |||||||
| Deleted: | ||||||||
| < < | List of Golden Gate Assembly subpages | |||||||
| Deleted: | ||||||||
| < < | ||||||||
| Changed: | ||||||||
| < < | Work in Progress. As of 12/14/2017, none of the above links are guaranteed to work or be accurate. This is a work in progress reorganization of the Barrick lab Golden Gate Assembly Protocols, including those used by the Microbe Hackers undergraduate research lab and UT Austin iGEM team. | |||||||
| > > | Supplies | |||||||
| Changed: | ||||||||
| < < | -Dr. Mishler | |||||||
| > > |
| |||||||
| Added: | ||||||||
| > > |
ProtocolUse the Calculator Spreadsheet (linked at the bottom of the page) for planning your reactions!
Golden Gate AssemblyGolden Gate Assembly - Start to Finish
References
Contributors
-- Main.KateElston - 29 Jan 2018
| |||||||
Revision 12017-12-14 - DennisMishler
|
View topic | History: r16 < r15 < r14 < r13 | More topic actions...